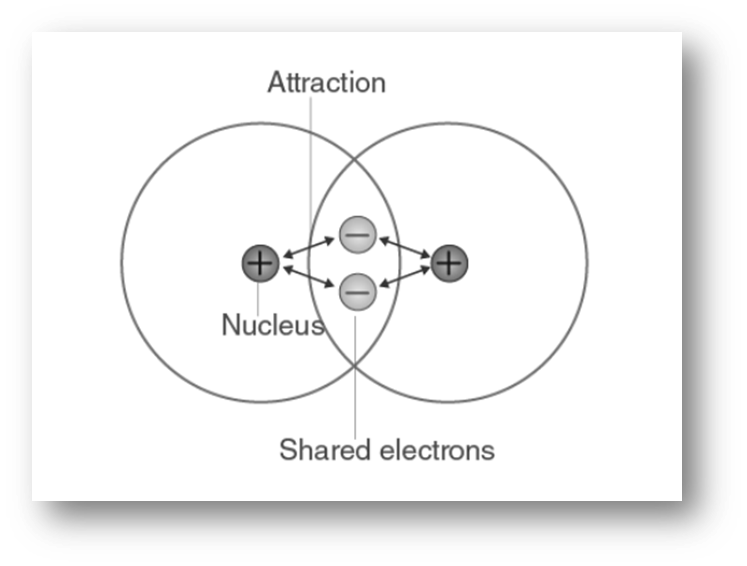
Appearance Of Covalent Compounds . A molecule is a group of atoms held together by covalent bonds. atomic orbitals (except for s orbitals) have specific directional properties leading to different types of covalent bonds. Atoms that share pairs of electrons form molecules. Sigma (σ) bonds are the strongest covalent bonds. covalent bonds form when two or more nonmetals combine. For example, both hydrogen and oxygen are nonmetals, and. Compounds that contain covalent bonds (also called molecular compounds). characteristics of covalent (molecular) compounds. covalent bonds between different atoms have different bond lengths and bond energies. covalent bonds are strong bonds. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. when looking at a compound and trying to determine whether it is an ionic compound or a. covalent compounds have bonds where electrons are shared between atoms.
from scienceinfo.com
atomic orbitals (except for s orbitals) have specific directional properties leading to different types of covalent bonds. covalent compounds have bonds where electrons are shared between atoms. Atoms that share pairs of electrons form molecules. Sigma (σ) bonds are the strongest covalent bonds. covalent bonds are strong bonds. For example, both hydrogen and oxygen are nonmetals, and. covalent bonds between different atoms have different bond lengths and bond energies. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. Compounds that contain covalent bonds (also called molecular compounds). characteristics of covalent (molecular) compounds.
Covalent Compounds Definition, Examples, Properties
Appearance Of Covalent Compounds Sigma (σ) bonds are the strongest covalent bonds. Compounds that contain covalent bonds (also called molecular compounds). Atoms that share pairs of electrons form molecules. when looking at a compound and trying to determine whether it is an ionic compound or a. covalent bonds form when two or more nonmetals combine. A molecule is a group of atoms held together by covalent bonds. atomic orbitals (except for s orbitals) have specific directional properties leading to different types of covalent bonds. characteristics of covalent (molecular) compounds. Sigma (σ) bonds are the strongest covalent bonds. covalent bonds between different atoms have different bond lengths and bond energies. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. covalent bonds are strong bonds. covalent compounds have bonds where electrons are shared between atoms. For example, both hydrogen and oxygen are nonmetals, and.
From ar.inspiredpencil.com
Covalent Compound Examples Appearance Of Covalent Compounds For example, both hydrogen and oxygen are nonmetals, and. atomic orbitals (except for s orbitals) have specific directional properties leading to different types of covalent bonds. covalent compounds have bonds where electrons are shared between atoms. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. Compounds that contain covalent bonds (also called molecular. Appearance Of Covalent Compounds.
From www.tes.com
Properties of Covalent Compounds Edexel 91 Teaching Resources Appearance Of Covalent Compounds covalent bonds form when two or more nonmetals combine. covalent compounds have bonds where electrons are shared between atoms. atomic orbitals (except for s orbitals) have specific directional properties leading to different types of covalent bonds. covalent bonds are strong bonds. For example, both hydrogen and oxygen are nonmetals, and. A molecule is a group of. Appearance Of Covalent Compounds.
From www.youtube.com
NAMING OF COVALENT COMPOUNDS NOMENCLATURE OF MOLECULAR COMPOUNDS Appearance Of Covalent Compounds covalent bonds are strong bonds. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. For example, both hydrogen and oxygen are nonmetals, and. covalent bonds between different atoms have different bond lengths and bond energies. covalent bonds form when two or more nonmetals combine. Atoms that share pairs of electrons form molecules.. Appearance Of Covalent Compounds.
From saylordotorg.github.io
Naming Covalent Compounds Appearance Of Covalent Compounds A molecule is a group of atoms held together by covalent bonds. covalent bonds are strong bonds. covalent bonds between different atoms have different bond lengths and bond energies. Compounds that contain covalent bonds (also called molecular compounds). covalent bonds form when two or more nonmetals combine. Atoms that share pairs of electrons form molecules. covalent. Appearance Of Covalent Compounds.
From ar.inspiredpencil.com
Covalent Compound Definition Appearance Of Covalent Compounds covalent bonds between different atoms have different bond lengths and bond energies. Compounds that contain covalent bonds (also called molecular compounds). For example, both hydrogen and oxygen are nonmetals, and. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. covalent compounds have bonds where electrons are shared between atoms. Sigma (σ) bonds are. Appearance Of Covalent Compounds.
From thechemistrynotes.com
Covalent Compounds Definition, Examples, Properties Appearance Of Covalent Compounds Atoms that share pairs of electrons form molecules. For example, both hydrogen and oxygen are nonmetals, and. characteristics of covalent (molecular) compounds. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. when looking at a compound and trying to determine whether it is an ionic compound or a. A molecule is a group. Appearance Of Covalent Compounds.
From fity.club
Molecular Shape Of Covalent Compound Appearance Of Covalent Compounds For example, both hydrogen and oxygen are nonmetals, and. Atoms that share pairs of electrons form molecules. Compounds that contain covalent bonds (also called molecular compounds). covalent bonds are strong bonds. A molecule is a group of atoms held together by covalent bonds. when looking at a compound and trying to determine whether it is an ionic compound. Appearance Of Covalent Compounds.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples Appearance Of Covalent Compounds covalent bonds form when two or more nonmetals combine. A molecule is a group of atoms held together by covalent bonds. For example, both hydrogen and oxygen are nonmetals, and. atomic orbitals (except for s orbitals) have specific directional properties leading to different types of covalent bonds. covalent compounds have bonds where electrons are shared between atoms.. Appearance Of Covalent Compounds.
From ar.inspiredpencil.com
Covalent Compound Examples Appearance Of Covalent Compounds Compounds that contain covalent bonds (also called molecular compounds). Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. when looking at a compound and trying to determine whether it is an ionic compound or a. For example, both hydrogen and oxygen are nonmetals, and. Sigma (σ) bonds are the strongest covalent bonds. characteristics. Appearance Of Covalent Compounds.
From spmchemistry.blog.onlinetuition.com.my
Covalent Bonding SPM Chemistry Appearance Of Covalent Compounds Atoms that share pairs of electrons form molecules. covalent compounds have bonds where electrons are shared between atoms. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. Compounds that contain covalent bonds (also called molecular compounds). For example, both hydrogen and oxygen are nonmetals, and. covalent bonds form when two or more nonmetals. Appearance Of Covalent Compounds.
From www.animalia-life.club
Covalent Compounds Appearance Of Covalent Compounds Sigma (σ) bonds are the strongest covalent bonds. A molecule is a group of atoms held together by covalent bonds. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. covalent compounds have bonds where electrons are shared between atoms. when looking at a compound and trying to determine whether it is an ionic. Appearance Of Covalent Compounds.
From sciencenotes.org
Naming Covalent Compounds Nomenclature Rules Appearance Of Covalent Compounds characteristics of covalent (molecular) compounds. covalent compounds have bonds where electrons are shared between atoms. Compounds that contain covalent bonds (also called molecular compounds). atomic orbitals (except for s orbitals) have specific directional properties leading to different types of covalent bonds. For example, both hydrogen and oxygen are nonmetals, and. covalent bonds are strong bonds. Atoms. Appearance Of Covalent Compounds.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Appearance Of Covalent Compounds characteristics of covalent (molecular) compounds. covalent bonds between different atoms have different bond lengths and bond energies. covalent compounds have bonds where electrons are shared between atoms. For example, both hydrogen and oxygen are nonmetals, and. Compounds that contain covalent bonds (also called molecular compounds). A molecule is a group of atoms held together by covalent bonds.. Appearance Of Covalent Compounds.
From www.showme.com
ShowMe Naming covalent compounds Appearance Of Covalent Compounds characteristics of covalent (molecular) compounds. For example, both hydrogen and oxygen are nonmetals, and. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. A molecule is a group of atoms held together by covalent bonds. Sigma (σ) bonds are the strongest covalent bonds. Atoms that share pairs of electrons form molecules. when looking. Appearance Of Covalent Compounds.
From www.slideshare.net
Covalent Bond Appearance Of Covalent Compounds atomic orbitals (except for s orbitals) have specific directional properties leading to different types of covalent bonds. when looking at a compound and trying to determine whether it is an ionic compound or a. characteristics of covalent (molecular) compounds. covalent bonds between different atoms have different bond lengths and bond energies. Sigma (σ) bonds are the. Appearance Of Covalent Compounds.
From studylib.net
Covalent Compounds Formula to Name Appearance Of Covalent Compounds covalent compounds have bonds where electrons are shared between atoms. Compounds that contain covalent bonds (also called molecular compounds). Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. when looking at a compound and trying to determine whether it is an ionic compound or a. Sigma (σ) bonds are the strongest covalent bonds.. Appearance Of Covalent Compounds.
From scienceinfo.com
Covalent Compounds Definition, Examples, Properties Appearance Of Covalent Compounds when looking at a compound and trying to determine whether it is an ionic compound or a. covalent bonds are strong bonds. A molecule is a group of atoms held together by covalent bonds. covalent bonds form when two or more nonmetals combine. atomic orbitals (except for s orbitals) have specific directional properties leading to different. Appearance Of Covalent Compounds.
From eduforkid.com
Covalent Compounds Examples At Home EduForKid Appearance Of Covalent Compounds Due to the sharing of electrons, they exhibit characteristic physical properties that include lower. For example, both hydrogen and oxygen are nonmetals, and. Compounds that contain covalent bonds (also called molecular compounds). covalent compounds have bonds where electrons are shared between atoms. characteristics of covalent (molecular) compounds. when looking at a compound and trying to determine whether. Appearance Of Covalent Compounds.